15 years one-stop China custom CNC machining parts factory
 285 |
Published by VMT at Aug 25 2025 | Reading Time:About 5 minutes
285 |
Published by VMT at Aug 25 2025 | Reading Time:About 5 minutes
When choosing materials for engineering, manufacturing, or CNC machining projects, one recurring question is whether nickel is magnetic. This is not a trivial detail—magnetic properties directly affect how metals behave in electrical components, sensors, motors, and even medical devices. Many engineers assume all metals that look silvery or conductive are magnetic, but this is not always true. Understanding the truth about nickel magnetism is essential, especially if you are sourcing nickel CNC machined parts for high-performance industries. At VMT CNC machining services, we often guide clients through selecting the right material based on not only strength and corrosion resistance but also magnetism. If you overlook this property, it can result in higher costs, design flaws, or compatibility issues with sensitive equipment.
Yes, nickel is magnetic. As a ferromagnetic metal, nickel can be attracted to magnets and even retain magnetism under certain conditions. Its magnetism depends on atomic structure, temperature, and alloying elements. This property makes nickel essential in magnetic alloys, sensors, motors, and CNC machined parts for advanced applications.
Now that we know nickel is indeed magnetic, it’s important to understand why it behaves this way and how its magnetism compares to other metals. We’ll also explore the conditions that strengthen or weaken nickel’s magnetic properties, and how this knowledge is applied in real-world industries—especially when choosing materials for CNC machining factories.
Nickel is a transition metal with atomic number 28, characterized by its silvery-white appearance, high corrosion resistance, and excellent durability. It is widely used in alloys such as stainless steel, Inconel, and Monel, which are essential in aerospace, automotive, electronics, and chemical processing. Pure nickel is relatively hard and ductile, making it suitable for nickel CNC machined parts that require both precision and resilience. Beyond mechanical properties, nickel’s ability to conduct electricity and resist oxidation makes it a valuable engineering material. Its role in electroplating and battery production further highlights its versatility. But the magnetic property of nickel—ferromagnetism—is what sets it apart in applications involving motors, transformers, and electronic devices.

Yes, nickel is magnetic. Specifically, it is a ferromagnetic material, meaning it is strongly attracted to magnets and can retain magnetism after being exposed to an external magnetic field. This makes nickel similar to iron and cobalt, which are also ferromagnetic. Nickel’s magnetic behavior is not superficial; it stems from its atomic electron configuration, where unpaired electrons create a strong magnetic moment. However, the intensity of nickel’s magnetism can vary depending on temperature, impurities, and structural conditions. For example, while pure nickel exhibits strong magnetism at room temperature, its behavior changes dramatically when heated beyond its Curie temperature (about 358 °C). Understanding this property is crucial when selecting materials for CNC machining services, especially for devices that operate in high-temperature or high-stress environments.

Nickel is magnetic because of its electron arrangement in the 3d and 4s orbitals. In simple terms, electrons carry a magnetic moment, and in nickel, several electrons remain unpaired. These unpaired electrons align in the same direction within regions called magnetic domains, creating a collective magnetic field. When exposed to an external magnetic field, these domains align further, enhancing nickel’s magnetism. This process makes nickel not only attracted to magnets but also capable of becoming a magnet itself. For engineers, this means that nickel-based CNC machined parts can interact with magnetic sensors or fields, a vital factor in industries such as robotics, medical imaging, and aerospace. If you need precision parts that require controlled magnetic behavior, working with an experienced CNC machining factory ensures proper material selection and heat treatment to optimize performance.
Yes, nickel can be a magnet, but only under specific conditions. As a ferromagnetic metal, nickel has the ability not only to be attracted to an external magnetic field but also to become magnetized itself. When exposed to a strong external magnetic field, the internal magnetic domains of nickel—regions where atomic magnetic moments align—can be reoriented so that they all point in the same direction. Once aligned, nickel can retain this orientation, meaning it has become a permanent magnet. However, compared to iron, which is the most commonly used magnetic material, nickel’s ability to hold magnetism is weaker.
The strength of nickel as a magnet also depends on temperature. Above its Curie temperature (358 °C or 676 °F), nickel loses its ferromagnetic properties and becomes paramagnetic, meaning it is only weakly attracted to magnets and cannot hold permanent magnetism. This limitation makes pure nickel less practical as a permanent magnet in high-temperature environments. Instead, nickel is often combined with other elements in alloys (such as Alnico or Permalloy) to enhance its magnetic stability and strength.
For industries relying on nickel CNC machined parts, this means that nickel can serve both structural and magnetic functions, but its use as a standalone permanent magnet is limited. In practice, nickel is more valuable in magnetic coatings, alloys, and components than as a pure magnet material. If your project requires permanent magnetism, consider nickel-based alloys rather than pure nickel for improved performance.
Nickel is classified as a ferromagnetic metal, but its behavior is not identical to other well-known ferromagnets such as iron and cobalt. Ferromagnetism is the property of certain metals where the magnetic moments of atoms align parallel to each other, resulting in strong magnetic attraction. Pure nickel exhibits this phenomenon at room temperature, which is why it is strongly attracted to magnets and can itself become magnetized. However, nickel’s ferromagnetism is slightly weaker than that of iron. This is because its atomic structure and electron configuration limit the maximum number of aligned unpaired electrons compared to iron, reducing its overall saturation magnetization.

Despite being a weaker ferromagnet than iron, nickel plays an essential role in modern magnetic applications. One of its most important uses is as a base material in magnetic alloys. For example, Permalloy (nickel-iron alloy) exhibits much stronger and more stable magnetic properties than pure nickel alone. These alloys are widely used in electromagnetic shielding, transformer cores, sensors, and recording heads because they balance strength, durability, and consistent magnetism.
Another important distinction is nickel’s resistance to corrosion compared to iron. While pure iron rusts quickly, nickel maintains its integrity even in harsh environments, making it suitable for nickel CNC machined parts in aerospace, marine, and chemical industries where both strength and controlled magnetism are required. This unique balance allows nickel to be applied in areas where iron cannot be used effectively, especially in high-performance electronics and energy systems.
For engineers and buyers working with CNC machining factories, the takeaway is clear: while pure nickel may not always be the strongest ferromagnetic option, its role in magnetic alloys and its corrosion resistance make it indispensable in advanced designs. Careful selection between pure nickel and nickel-based ferromagnetic alloys ensures optimal performance for your project’s requirements.
| Aspect | Nickel Metal | Ferromagnetism in Nickel |
|---|---|---|
| Definition | A transition metal (atomic number 28) with silvery-white appearance, high corrosion resistance, and strength. | A magnetic property where atomic magnetic moments align parallel, creating strong magnetism. |
| Cause | Electron configuration with unpaired electrons in the 3d orbital. | Alignment of magnetic domains within the crystal lattice. |
| Magnetic Strength | Naturally ferromagnetic but weaker than iron. | Strong attraction to external fields; can become magnetized itself. |
| Temperature Sensitivity | Stable at room temperature. | Loses ferromagnetism above the Curie temperature (358 °C / 676 °F). |
| Industrial Use | Structural parts, coatings, corrosion-resistant alloys, and nickel CNC machined parts. | Magnetic alloys (Permalloy, Alnico), shielding, sensors, recording media, and transformers. |
| Comparison to Iron | More corrosion-resistant, but less strongly magnetic. | Forms alloys with iron to enhance magnetic properties. |
This table simplifies how nickel as a metal differs from its behavior as a ferromagnetic material, making it easier to understand why it’s valued in both structural and magnetic applications.
Nickel’s magnetism originates from its atomic structure—specifically, the arrangement of its electrons. In atoms, electrons orbit the nucleus in different energy levels or shells. Each shell contains subshells (s, p, d, f), and the 3d subshell of nickel is especially important for magnetism. Nickel has an electron configuration of [Ar] 3d⁸ 4s². This means that in the 3d orbital, there are eight electrons, with two of them left unpaired. These unpaired electrons generate magnetic moments, tiny magnetic fields associated with the spin of electrons.
In most metals, these magnetic moments cancel each other out because electrons are paired, spinning in opposite directions. However, in nickel, because of its unpaired electrons, many of the atomic magnetic moments can align in the same direction. When large groups of atoms within nickel align their magnetic moments, they form regions called magnetic domains. Each domain acts like a small magnet. Under normal conditions, these domains are randomly oriented, so nickel doesn’t appear strongly magnetic. But when exposed to an external magnetic field, the domains align, causing nickel to exhibit strong ferromagnetism.
Another factor is the exchange interaction, a quantum mechanical effect that favors the parallel alignment of neighboring electron spins. This interaction makes it energetically stable for nickel atoms to align their magnetic moments in the same direction, reinforcing ferromagnetism.
This atomic-level behavior explains why nickel is not just weakly attracted to magnets (like paramagnetic materials such as aluminum) but can actually retain magnetism and become a magnet itself. For engineers working with nickel CNC machined parts, understanding this is critical: at the atomic level, nickel’s magnetism is both a design advantage and a factor that must be carefully controlled in high-precision applications such as sensors, motors, and electronic devices.
Nickel belongs to a small but important group of ferromagnetic metals, which also includes iron and cobalt. While all three share the ability to be strongly attracted to magnets and retain magnetism, they differ significantly in strength, stability, and industrial applications. For engineers and designers working with CNC machining services, knowing these differences is critical when selecting the right metal for motors, transformers, sensors, or precision nickel CNC machined parts. The following sections break down these differences in terms of Curie temperature, magnetic permeability, and practical uses.
Curie Temperature Difference
The Curie temperature is the point at which a ferromagnetic material loses its permanent magnetism and becomes paramagnetic. This factor is crucial for high-temperature applications such as aerospace, automotive, and power generation.
Thus, while nickel is magnetic at room temperature, it loses magnetism faster than iron or cobalt under heat, which is why it is rarely used as a standalone permanent magnet in demanding environments.
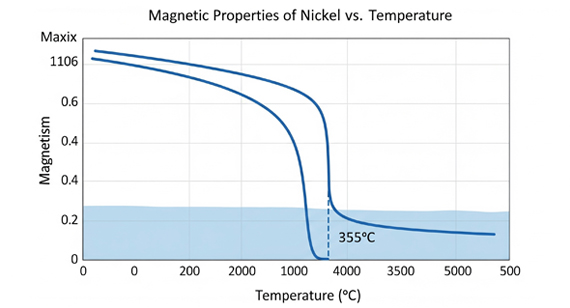
Permeability Properties
Magnetic permeability measures how easily a material can become magnetized in the presence of an external field.
Nickel’s role here is often as an enhancer in alloys rather than as a pure material, allowing precise tuning of permeability to meet specific industrial needs.
Practical Applications of Different Magnetic Metals
Each ferromagnetic metal has distinct industrial applications based on its strengths:
For manufacturers, nickel’s biggest contribution lies in its role as an alloying element that enhances corrosion resistance and tunes magnetic properties, rather than serving as the strongest magnet on its own.
Table: Comparison of Nickel, Iron, and Cobalt in Magnetism
| Property |
Nickel (Ni) |
Iron (Fe) |
Cobalt (Co) |
| Magnetic Type |
Ferromagnetic | Ferromagnetic | Ferromagnetic |
| Curie Temperature |
~358 °C (676 °F) | ~770 °C (1420 °F) | ~1,115 °C (2,039 °F) |
| Relative Magnetic Strength |
Moderate – weaker than iron | Strong – highest practical magnetism | Strong but less than iron |
| Permeability |
Moderate; enhanced in alloys like Permalloy | Very high; ideal for electromagnets and cores | Lower than iron, but stable at high heat |
| Corrosion Resistance |
High – resists oxidation and harsh environments | Low – rusts easily unless alloyed | Moderate – better than iron but less than nickel |
| Industrial Role |
Used in nickel CNC machined parts, sensors, magnetic shielding, rechargeable batteries, and high-performance alloys | Widely used in motors, transformers, generators, and magnetic storage | Used in aerospace, defense, permanent magnets, and high-temperature magnetic systems |
| Cost & Availability |
More expensive than iron; widely available | Low cost, abundant | More expensive, less abundant |
This table makes it clear that while nickel is not the strongest magnetic metal, its corrosion resistance and role in alloys make it uniquely valuable for advanced engineering and CNC machining services.
While nickel is naturally ferromagnetic, its magnetic behavior is not fixed. Instead, it depends on a variety of physical, chemical, and environmental conditions. For engineers and manufacturers, understanding these influences is vital, especially when designing nickel CNC machined parts for sensitive equipment such as sensors, motors, and electromagnetic devices. Small changes in temperature, impurities, or mechanical stress can significantly alter how nickel responds to magnetic fields. These factors determine whether nickel should be used in its pure form, as an alloy, or treated to enhance or suppress its magnetism. Ignoring them could result in reduced efficiency, higher energy consumption, or increased machining costs.
Crystal Structure
Nickel’s magnetic properties are strongly tied to its face-centered cubic (FCC) crystal structure. In this arrangement, atoms pack closely together, allowing unpaired electrons in the 3d orbitals to interact more efficiently. This interaction stabilizes the alignment of electron spins, producing ferromagnetism. However, if the crystal structure is altered—through alloying, defects, or rapid cooling—the magnetic behavior can weaken. For instance, nickel-based superalloys used in aerospace may lose some ferromagnetism because additional elements distort the crystal lattice.
Temperature
Temperature plays a decisive role in nickel magnetism. At room temperature, nickel is ferromagnetic. However, once it is heated beyond its Curie temperature (358 °C / 676 °F), it transitions to a paramagnetic state. In this state, nickel is still weakly attracted to magnetic fields but cannot retain permanent magnetism. This transition is critical for applications in motors, turbines, and high-temperature electronics, where exceeding the Curie point could compromise performance.
Magnetic Domains
Nickel’s magnetism originates from magnetic domains—microscopic regions where atoms’ magnetic moments are aligned. In an unmagnetized nickel sample, these domains are randomly oriented, canceling out overall magnetism. When an external magnetic field is applied, the domains align, and the nickel becomes strongly magnetic. The stability of these domains depends on factors like impurities, stress, and grain boundaries. Engineers often manipulate domain structures to enhance or suppress magnetic performance, depending on the application.
Impurities and Alloys
Pure nickel is ferromagnetic, but when combined with other elements, its magnetism can change dramatically. For example:
This shows how CNC machining factories can customize material properties for a client’s exact needs by selecting the right alloy composition.
Mechanical Stress
Mechanical stress—such as stretching, bending, or machining operations—can alter nickel’s magnetic behavior. Stress changes the distance between atoms in the crystal lattice, which in turn affects electron alignment and magnetic domain stability. For example, stressed nickel may exhibit lower magnetic permeability or lose some magnetization. This is particularly important in precision nickel CNC machined parts, where tight tolerances and repeated stress cycles may gradually weaken magnetism.
Granularity
The size and distribution of nickel grains in a material also influence magnetism. Fine-grained nickel may have more stable magnetic properties because domain walls encounter more barriers at grain boundaries. Conversely, coarse grains allow domains to move more freely, which can lead to inconsistent magnetism. Controlling grain size through processing techniques such as annealing allows engineers to tune nickel’s performance for specific magnetic applications.
External Magnetic Field
Nickel’s magnetism increases in the presence of an external magnetic field. A strong applied field forces magnetic domains to align, maximizing nickel’s ferromagnetism. However, once the external field is removed, nickel may retain some magnetism (remanence), depending on its treatment and purity. This property is essential in designing magnetic sensors, memory devices, and shielding applications.
Pressure
High pressure can influence the electronic structure of nickel, potentially altering its magnetic properties. Under extreme compression, the spacing between atoms changes, affecting electron spin alignment and weakening ferromagnetism. While this effect is less relevant in everyday environments, it becomes critical in aerospace, defense, and deep-sea engineering, where parts face high-pressure conditions. For nickel CNC machined parts in such industries, considering pressure effects ensures long-term reliability.
Table: Factors Affecting Nickel’s Magnetism
| Factor |
Description |
Effect on Nickel Magnetism |
| Crystal Structure |
Nickel has a face-centered cubic (FCC) lattice that supports ferromagnetism. | Distortions or alloying can weaken magnetic alignment. |
| Temperature |
Below the Curie temperature (358 °C / 676 °F), nickel is ferromagnetic. | Above Curie point, it becomes paramagnetic and loses permanent magnetism. |
| Magnetic Domains |
Microscopic regions where atomic magnetic moments align. | External fields align domains, increasing magnetism; random orientation reduces it. |
| Impurities & Alloys |
Alloying with Fe enhances magnetism (Permalloy), while Cu or Cr reduce it. | Magnetic strength can either increase or disappear, depending on alloy. |
| Mechanical Stress |
Stress alters atomic spacing and domain stability. | May decrease permeability and weaken magnetism. |
| Granularity |
Refers to grain size in the metal’s microstructure. | Fine grains stabilize domains; coarse grains cause inconsistent magnetism. |
| External Magnetic Field |
Strong applied field aligns magnetic domains. | Increases magnetism; residual magnetism may remain after removal. |
| Pressure |
High external pressure changes atomic spacing and electron spin alignment. | Can weaken ferromagnetism under extreme conditions. |
This table provides a quick reference for engineers and buyers, making it easier to predict how nickel CNC machined parts will behave in different environments.
The Curie point (or Curie temperature) of nickel is approximately 358 °C (676 °F). This is the critical temperature above which nickel loses its ferromagnetic properties and becomes paramagnetic. In the ferromagnetic state, nickel’s magnetic domains are aligned, giving it strong, permanent magnetism. Once the Curie temperature is exceeded, the thermal energy is high enough to disrupt this alignment, causing the domains to randomize. As a result, nickel can still be weakly attracted to magnetic fields, but it can no longer retain magnetism on its own.
This transition has significant engineering and industrial implications. For example, in electrical motors, sensors, and transformers, nickel’s magnetic properties are essential for efficiency. If these devices operate at temperatures close to or above the Curie point, nickel components may lose their effectiveness, leading to reduced performance or even failure. In contrast, nickel alloys like Permalloy (Ni-Fe) or cobalt-based materials are preferred in high-temperature environments because they maintain magnetism at higher thresholds.
For manufacturers relying on nickel CNC machined parts, knowing the Curie temperature is critical when designing components exposed to heat. Parts used in aerospace turbines, automotive exhaust systems, or high-power electronics may need either protective cooling systems or substitution with alloys that preserve magnetic strength under extreme conditions. Ignoring this factor could result in higher costs from replacements, reduced efficiency, or safety risks.
Here’s a clear comparison table for Nickel’s Curie Point (Temperature):
| Property |
Nickel (Ni) |
Iron (Fe) |
Cobalt (Co) |
Note |
| Curie Temperature (°C) |
~358 °C | ~770 °C | ~1,115 °C | Nickel has the lowest Curie point among common ferromagnetic metals. |
| Curie Temperature (°F) |
~676 °F | ~1,418 °F | ~2,039 °F | Important for high-temperature applications. |
| Magnetic Behavior Below Tc |
Ferromagnetic | Ferromagnetic | Ferromagnetic | Strong domain alignment enables permanent magnetism. |
| Magnetic Behavior Above Tc |
Paramagnetic | Paramagnetic | Paramagnetic | Only weak, induced magnetism remains. |
| Industrial Relevance |
Limited for high-heat uses | Better for moderate-heat uses | Best for extreme-heat uses | Engineers select based on working temperature range. |
This table shows that nickel loses ferromagnetism much earlier than iron or cobalt, which is why in high-temperature environments (motors, turbines, aerospace), nickel is often combined with other metals to form alloys with higher Curie points.
Nickel’s ferromagnetism, electrical conductivity, and resistance to corrosion make it essential in numerous industries. Its magnetic behavior is leveraged in permanent magnets, recording media, electromagnetic devices, and shielding applications. Often, nickel is used alone or in alloys to optimize strength, temperature stability, and magnetization.

1. Permanent Magnets
2. Magnetic Alloys
3. Magnetic Recording Media
4. Electromagnetic Components
5. Magnetic Sensors
6. Microwave Devices
7. Electrical Motors
8. Transformers and Inductors
9. Magnetic Shielding Applications
10. Energy Storage and Battery Technology
11. Electronic Components and Data Storage
12. Medical and Scientific Applications
13. Industrial Sensing and Control
In short: Nickel’s magnetism is not just about permanent magnets—it powers technologies in electronics, energy, medical, and industrial fields.
Here’s a structured table for Practical Applications of Nickel’s Magnetism:
| Application Area |
Role of Nickel’s Magnetism |
Examples / Use Cases |
| Permanent Magnets |
Enhances strength and stability of magnets when alloyed with other metals | Alnico magnets, samarium–cobalt magnets |
| Magnetic Alloys |
Provides ferromagnetic properties, corrosion resistance, and toughness | Permalloy (Ni-Fe), Mu-metal (Ni-Fe-Mo-Cu) |
| Magnetic Recording Media |
Used in thin-film coatings for storing digital information magnetically | Hard disk drives, magnetic tapes |
| Electromagnetic Components |
Improves magnetic cores with low losses and high permeability | Relay cores, solenoids, inductors |
| Magnetic Sensors |
Utilized in alloys for sensitive detection of magnetic fields | Hall-effect sensors, magnetoresistive sensors |
| Microwave Devices |
Nickel alloys stabilize magnetic permeability at high frequencies | Microwave isolators, circulators |
| Electrical Motors |
Provides efficiency and torque stability in high-performance motors | Industrial motors, EV motors |
| Transformers & Inductors |
Reduces energy losses through high magnetic permeability | Power transformers, RF inductors |
| Magnetic Shielding |
Blocks unwanted magnetic interference (EMI/RFI shielding) | Electronic enclosures, MRI rooms |
| Energy Storage & Batteries |
Nickel contributes to electrochemical & magnetic stability | NiMH batteries, nickel–cadmium batteries |
| Electronic Components & Data Storage |
Used in magnetic coatings and memory storage devices | Magnetic RAM (MRAM), HDDs |
| Medical & Scientific Applications |
Nickel alloys used for magnetic shielding and precision instruments | MRI machines, NMR spectrometers |
| Industrial Sensing & Control |
Provides precision in automation and industrial measurement systems | Proximity sensors, process monitoring |
This table shows how nickel’s ferromagnetic properties make it a versatile material across industries — from electronics and motors to medical imaging and data storage.
Nickel is one of the few naturally ferromagnetic metals, and its magnetic properties make it indispensable across science, technology, and industry. From its Curie temperature of 355 °C to its role in alloys like Permalloy and Mu-metal, nickel demonstrates a unique balance of strength, stability, and versatility. Compared with other magnetic metals such as iron and cobalt, nickel provides superior resistance to corrosion, excellent permeability, and consistent performance in demanding environments.
Its magnetism has been harnessed in countless applications: permanent magnets, electromagnetic components, transformers, motors, sensors, recording media, and medical devices. Whether in energy storage, shielding sensitive equipment, or enabling data storage technologies, nickel’s contribution remains vital to modern life.
As industries move toward miniaturization, higher efficiency, and advanced energy solutions, nickel will continue to play a central role. Its magnetic behavior, combined with its chemical stability, ensures that nickel is not just a traditional industrial metal but a critical enabler of future technologies.
Can Nickel Be Magnetized?
Yes. Pure nickel is a ferromagnetic material, meaning it can be magnetized when exposed to an external magnetic field and retain some magnetism afterward.
Are Nickel Alloys Magnetic?
Some nickel alloys (like Permalloy and Mu-metal) are highly magnetic with excellent permeability, while others (such as certain stainless steels containing nickel) are non-magnetic depending on composition and crystal structure.
How Does Temperature Affect Nickel's Magnetism?
Nickel loses its ferromagnetic properties above its Curie temperature of about 355 °C (671 °F). Below this point, it remains magnetic, but its strength can vary with temperature changes.
Can Nickel Be Used to Make Permanent Magnets?
Yes, though nickel alone is not the strongest option. It is often combined with iron, cobalt, or rare earths to create stronger and more stable permanent magnets.
What is the Curie Temperature of Nickel?
The Curie temperature of nickel is approximately 355 °C (671 °F).
Can a Magnet Attract Nickel?
Yes. Nickel is strongly attracted to magnets due to its ferromagnetic properties.
How Can I Test Whether a Metal Is Nickel?
You can test with a magnet—nickel will be attracted. Additional verification can be done with chemical spot tests, density measurements, or X-ray fluorescence (XRF) analysis.
Is 100% Nickel Magnetic?
Yes. Pure nickel is magnetic at room temperature and below its Curie point.
Can Nickel Rust?
Nickel does not rust like iron, but it can corrode in certain conditions. It forms a protective oxide layer that improves corrosion resistance, which is why it is often used in alloys and coatings.
What Is the Most Magnetic Metal?
Iron is considered the most strongly magnetic metal in everyday use, followed closely by cobalt and nickel.
How Do Different Alloys Affect the Magnetic Behavior of Nickel?
Alloying can either enhance or reduce nickel’s magnetism. For example, adding iron can strengthen it (as in soft magnetic alloys), while combining with chromium (as in some stainless steels) can suppress its magnetic properties.
