15 years one-stop China custom CNC machining parts factory
 68 |
Published by VMT at Nov 18 2025 | Reading Time:About 2 minutes
68 |
Published by VMT at Nov 18 2025 | Reading Time:About 2 minutes
Plastic materials are widely used in manufacturing plastic parts in engineering. The selection of plastic materials depends on the required material properties , that is, the properties of the plastic itself. So, when you select the plastic materials for your projects , have you ever wondered the causes of the plastic’s properties in chemistry?
This article will share you knowledge of copolymers and homopolymers. With examples of four common plastic materials, you will understand what properties plastics have when they are copolymers or homopolymers.
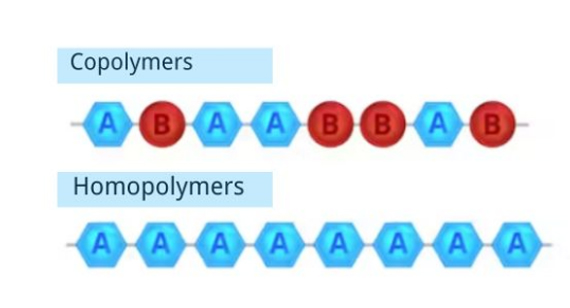
How Do We Define Copolymers and Homopolymers?
As the diagram shows, copolymers are combinations of different types of bonded monomers. Homopolymers are combinations of one type of monomers.
Difference Between Copolymers and Homopolymers
For the copolymers
More than one type of bonded monomers in copolymers making the copolymers achieve the balance of plastics properties. Different types of bonded monomers alter the properties of the plastic.
For the homopolymers
Homopolymers exhibit the properties more leanly influenced by that type of monomers, which means the main chain of the structure.
Table 1: Comparision of Copolymers and Homopolymers
| Properties/Type |
Copolymers |
Homopolymers |
| Polymerization |
Condensation polymerization | Addition polymerization |
| Cystal structure |
Less stable | Stable |
| Reactivity |
Balance | Leaning to the main chain |
| Tensile strength(generic) |
60 MPa | 69 MPa (Better) |
| Stiffness |
/ | / |
| Impact strength |
/ | / |
| Initial creep resistance |
/ | Better |
| Thermal expansivity |
Usually higher | / |
| Dimensional stability |
Long term | Short term |
| Surface energy |
Usually better(if there’re polar groups) | / |
| Chemical stability |
/ | / |
To further disscuss the specific properties of plastics when they are copolymers or homopolymers, I will illustrate with examples of ABS, POM, PEEK, and PTFE in the following.
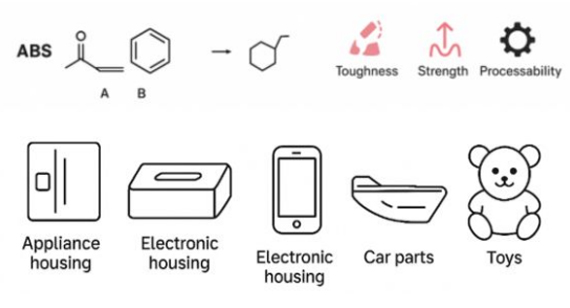
ABS stands for Acrylonitrile-Butadiene-Styrene. It is a copolymer of three monomers: Acrylonitrile (A), Butadiene (B), and Styrene (S), polymerized using an emulsifier as a medium.
Impact of Polymerization on ABS
The interactions among acrylonitrile (A), butadiene (B), and styrene (S), as well as their relative ratios, affect the properties of ABS. (Ratios are changable).
Table 2: Impact of Polymerization on ABS
| Aspect |
Chemical Formula |
Positive Impact |
Negative Impact |
| Acrylonitrile (A) |
C₃H₃N |
|
— |
| Butadiene (B) |
C₄H₆ |
|
|
| Styrene (S) |
C₈H₈ |
|
— |
| Copolymer Effects (ABS overall) |
— |
|
|
Typical Applications of Copolymer ABS
Combinations of three types of monomers in ABS achieves a balance of strength, toughness, and processability, making it suitable for:
Polyoxymethylene (POM) exists in both copolymer and homopolymer forms. POM-C is a copolymer, while POM-H is a homopolymer.
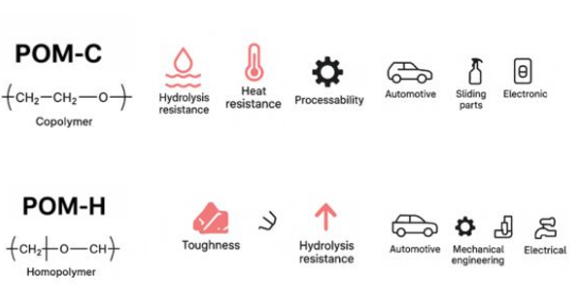
Impact of Polymerization on POM-C and POM-H
Table 3: Impact of Polymerization on POM-C and POM-H
| Aspect |
Copolymer POM-C |
Homopolymer POM-H |
| Chemical Formula |
Copolymer of formaldehyde (CH₂O)and a small amount ofethylene oxide (C₂H₄O) | Produced from formaldehyde (CH₂O)ortrioxymethylene (C₃H₆O₃); repeating unit–CH₂–O– |
| Trait of the Structure |
Slightly less crystalline due to random incorporation of ethylene oxide | Main chain is more regular and highly crystalline |
| Positive Impact |
|
|
| Negative Impact |
|
|
The copolymer POM-C has a small amount of ethylene oxide participating in the reaction, which compensates for the poor thermal stability of the homopolymer POM-H, making the copolymer POM-C have better resistance to heat aging and chemical resistance. However, the trade-off is a slight decrease in crystallinity and a slight sacrifice in strength.
The homopolymer POM-H has a more regular main chain, resulting in higher crystallinity, higher strength, and better wear resistance.
Typical Applications of POM-C and POM-H
POM-C
POM-H
PEEK stands for Polyether ether ketone, formed by the polymerization of a specific monomer sequence (p-Hydroxyacetophenone), and is a highly ordered linear homopolymer.
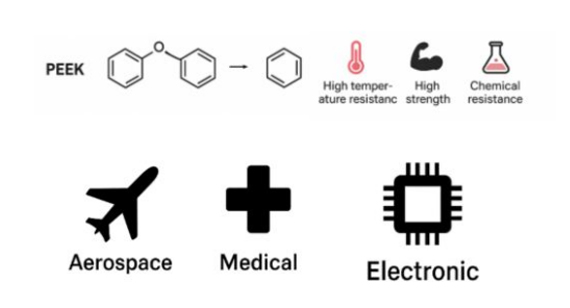
Impact of Polymerization on PEEK
Properties of PEEK lean to it’s linear main chain( –O–Ph–O–Ph–CO–Ph–).
Table 4: Impact of Polymerization on PEEK
| Aspect |
PEEK (Polyetheretherketone) |
| Chemical Formula |
Homopolymer synthesized by polycondensation of p-hydroxyacetophenone; repeating unit –O–Ph–O–Ph–CO–Ph– |
| Trait of the Structure |
|
| Positive Impact |
|
| Negative Impact |
|
The hydroxyl and carbonyl groups of p-Hydroxyacetophenone are highly reactive, allowing it to participate in polycondensation reactions as a monomer to produce PEEK. Therefore, the PEEK backbone exhibits a highly aromatic structure: –O–Ph–O–Ph–CO–Ph–.
The PEEK structure contains alternating rigid aromatic rings and flexible ether bonds. The rigid aromatic rings contribute to its high rigidity, high initial creep resistance, high thermal oxidation stability, heat resistance, and chemical corrosion resistance.
The rigid backbone of homopolymer PEEK makes it generally brittle, with low impact strength, resulting in poor processability.Although the flexible ether bonds can provide some flexibility and processability to PEEK, this flexibility and processability are actually limited.
Typical Applications of Homopolymer PEEK
PTFE (Polytetrafluoroethylene) is formed by the polymerization of one type of monomer (tetrafluoroethylene).
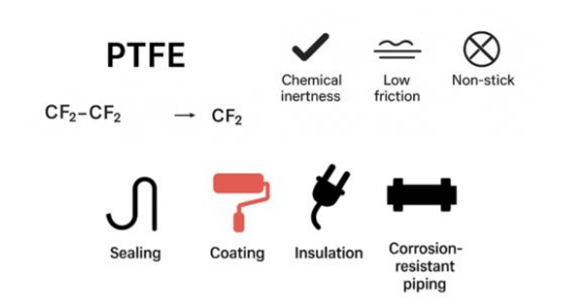
Impact of Polymerization on PTFE
The chemical formula for tetrafluoroethylene is CF₂=CF₂, and the main chain of PTFE, which is polymerized from it, is –CF₂–CF₂–.The strong C–F bonds from polymerization, along with the smooth molecular chains and non-polar nature, impact the properties of PTFE.
Table 5: Impact of Polymerization on PTFE
| Aspect |
Description |
| Chemical Formula |
Monomer: Tetrafluoroethylene (CF₂=CF₂) Polymer backbone: –CF₂–CF₂– |
| Trait of the Structure |
Strong covalent C–F bonds in the polymer chain smooth molecular chains |
| Positive Impact |
Excellent chemical inertness and heat resistance due to strong C–F bonds Outstanding electrical insulation because of tightly bound electrons Very low coefficient of friction (“self-lubricating”) Extremely low surface energy, giving non-stick properties |
| Negative Impact |
Poor processability – cannot be melt-processed, must be sintered at high temperatures (>327 °C) Low flexibility and inability to recover after deformation High thermal expansion, leading to poor dimensional stability Prone to permanent deformation under stress due to non-polar structure |
Typical Applications of Homopolymer PTFE
Homopolymer PTFE possesses excellent chemical resistance, high temperature resistance, low coefficient of friction, and outstanding insulation properties, making it ideal for sealing, insulation, and corrosion-resistant applications.
Typical applications include:
A deeper understanding of plastics can help you make the choices of plastic materials for your project. If you have questions about certain plastic material, VMT is here to assist.
VMT is a specialized plastic prototyping and CNC machining factory, dedicated to delivering high-precision custom plastic parts services for diverse industries. With advanced CNC equipment, strict quality control systems, and expert surface finishes, VMT ensures every plastic part meets exact dimensional and surface finish specifications. If you need professional advice on selecting materials for customized plastic parts, welcome to contact us. We are available 24/7.

What are other examples of copolymers?
Other common copolymers include styrene-butadiene rubber (SBR), high-impact polystyrene (HIPS), Nylon 6/6.
What are other examples of homopolymers?
Other common homopolymers include polyethylene (PE), and polyvinyl chloride (PVC).
Is polypropylene(PP) a copolymer or a homopolymer?
Polypropylene (PP) can be both a homopolymer and a copolymer. Homopolymer polypropylene (PPH) is polymerized from a single propylene monomer, while copolymer polypropylene (PPC) is copolymerized from propylene monomer with a small amount of ethylene monomer.
Which has a higher density, copolymer or homopolymer?
Both copolymers and homopolymers have a generic density of 0.9 g/cm³, so they have the same density.
Which has higher strength, copolymer or homopolymer?
In terms of general tensile strength, copolymer has 60 MPa, while homopolymer has 69 MPa. Therefore, homopolymer has higher strength and higher crystallinity.
