15 years one-stop China custom CNC machining parts factory
 67 |
Published by VMT at Nov 28 2025 | Reading Time:About 2 minutes
67 |
Published by VMT at Nov 28 2025 | Reading Time:About 2 minutes
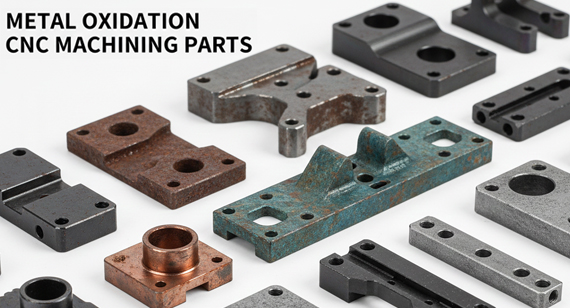
Metal oxidation is a common phenomenon in your metal devices or parts. If you’re wondering how to maintain metal surfaces well, ensuring your metal parts lifespan in your products and your customers satisfaction, you should understand metal oxidation.
This article will guide you through the causes and prevention methods of metal oxidation (including some professional surface treatment methods). Whether you're an individual needing metal parts maintenance or a manufacturer seeking metal oxidation surface finishes solutions, this article will be helpful to you.
Metal oxidation refers to the process by which a metal loses electrons. When a metal comes into contact with oxygen or other oxidizing agents (such as water, salt, and other chemical contaminants), a chemical change typically occurs on its surface.
In CNC machined parts, metal oxidation most commonly manifests as:
1. Surface darkening
2. Formation of discoloration or yellowing spots
3. Rusting and pitting
4. Reduced surface finish
5. Loss of protective film
However, metal oxidation itself is not always a bad thing. For example, aluminum naturally forms an oxide film to provide protection.But uncontrolled oxidation can lead to a decrease in appearance, performance, and structural strength.
Metal oxidation vs. Rust vs. Corrosion: What Are the Differences Between?
Have you been confused by the conception of metal oxidation, rusting and corrosion?
Rusting of Metals
Metal rust (such as iron rust) is a common form of metal corrosion. Essentially, it's the electrochemical oxidation of iron in a humid, oxygen-rich environment. Therefore, rust is both a form of metal corrosion and a form of metal oxidation.

Metal Corrosion
Metal corrosion is the reaction between a metal and its surrounding environment; it's a broader process of material deterioration, encompassing oxidation, pitting, and electrochemical corrosion. Metal corrosion includes metal oxidation, but

Metal Oxidation
Metal oxidation is a chemical process in which a metal loses electrons and its valence state increases. It is a broader concept than corrosion, encompassing oxidation that naturally forms an oxide film, as well as chemical oxidation reactions and electrochemical corrosion (such as rusting). Therefore, rusting and most forms of corrosion belong to metal oxidation, but metal oxidation has a wider scope.
The rate of metal oxidation is closely related to the environment, the material itself, and processing methods. Understanding these factors will help you better understand how to fundamentally prevent metal oxidation.
Environmental Factors
1.Oxygen and Humidity (Key Conditions): Metals exposed to high humidity and high oxygen content will oxidize more quickly.
2.Acidic/Alkaline Environments: For example,metal exposed to acid rain will accelerate the oxidation of metals.
3.Salinity: High-salinity environments, such as seawater, accelerate rusting or corrosion, and speed up metal oxidation.
4.Temperature Changes: High-temperature environments generally accelerate the metal oxidation reaction faster than low-temperature environments.
Material Selection
Improper Processing
1. Residual cutting fluid causes "yellow spots" on aluminum parts.
2. Lack of post-sandblasting treatment causes aluminum parts to darken rapidly.
3. Residual cleaning agents cause "brown spots" on stainless steel.
4.Unprotected direct contact with air leads to oxidation of polished parts.
Here I list some examples of metal oxidation based on the reaction mechanism for you to check.
Table 1: Examples of Metal Oxidation Based on the Reaction Mechanism
| Typical examples |
Mechanism |
Belong to |
Prevention |
| Aluminum (Al₂O₃), Stainless steel (Cr₂O₃), Titanium (TiO₂) |
Metals form a protective passivation film with oxygen | Natural oxidation | Passivation, anodizing, material selection |
| Rust, silver sulfide, verdigris, zinc oxide |
Metals oxidize with O₂, H₂O, salts, and contaminants | Single-metal chemical reaction | Coating, plating, anti-tarnishing agents, inhibitors, drying |
| Aluminum-copper, steel-brass corrosion, ship hull corrosion |
Two metals + electrolyte → form a corrosion cell. | Multi-metal electrochemical reaction (galvanic corrosion) | Cathodic protection, electrical isolation, galvanizing, appropriate material selection. |
Metal oxidation can cause structural damage, performance degradation, and aesthetic deterioration in metal parts, increasing maintenance costs. Furthermore, even slight surface oxidation can affect the fitting accuracy and functionality of CNC parts.
Here are some examples of common metal oxidation phenomena and their effects.
Table: Common metal oxidation phenomena and their effects.
| Materials |
Oxidation Performance |
Effects |
| Aluminum (Al₂O₃) |
Darkening, yellowing spots | Appearance deterioration, increased assembly friction |
| Stainless Steel (Cr₂O₃ film) |
Tea spots, pitting | Localized corrosion, reduced lifespan |
| Iron/Steel (Fe₂O₃) |
Rust | Decreased strength, structural failure |
| Copper, Brass |
Discoloration, verdigris | Appearance changes, potentially affecting contact performance |
| Magnesium Alloy |
Powdering | Rapid failure, high risk |
Structural Damage
Performance Degradation
Appearance Deterioration
Cost Impact
In industrial production, machinery manufacturing, electronic equipment, automotive, and aerospace fields, effectively preventing metal oxidation is a key technology for ensuring product performance and lifespan.
You can improve the corrosion resistance and stability of metal parts and slow down metal oxidation by addressing multiple aspects, including environmental factors, material selection, surface treatment techniques, and chemical methods.
You can choose to use rust-proof metals, corrosion-resistant metals or alloys, and avoid using reactive metals in highly corrosive environments.
2. Environmental Control
You may keep metal surfaces dry and reduce contact between metals and corrosive substances such as sulfides.
3. Chemical Inhibition
Chemical inhibitors adsorb onto the metal surface can reduce reactivity.

Proper surface treatment can significantly extend the lifespan of parts.
5. Advanced Surface Protection Technologies
In the production of precision parts, surface treatment of metal materials is a crucial step in preventing metal oxidation, improving appearance, and extending service life. Different metal materials have different compositions and properties, and therefore require different surface treatment methods. The following are typical surface treatment solutions for common metal materials for you to check.
Table: Commonly used metal materials and surface treatment processes
| Metal Category |
Common Material Examples |
Typical Surface Finishes |
| Aluminum & Aluminum Alloys |
6061, 6063, 7075, 5052 | Anodizing, Hard Anodizing, Coating/Painting, Chemical Conversion (Chromate/Non-chromate) |
| Stainless Steel |
304, 316, 316L, 430, 410 | Passivation, Electropolishing, Brushing, Mirror Polishing |
| Carbon Steel / Alloy Steel |
Q235, Q345, 45#, 40Cr, 35CrMo | Zinc Plating (Cold/Hot Dip), Nickel Plating, Phosphating, Painting, Black Oxide |
| Copper & Copper Alloys |
H62, H65, T2 Copper, Bronze (QSn) | Anti-oxidation Treatment, Cleaning + Sealant, Electroplating (Nickel/Silver/Tin) |
| Magnesium Alloys |
AZ91D, AM60B | Ceramic Coating (MAO), Painting, Sealing Treatment, Chemical Conversion |
| Titanium & Titanium Alloys |
TA1, TA2, TC4 (Ti-6Al-4V) | Anodizing (Color), Polishing, Sandblasting, PVD Coating |
| Zinc Alloys |
Zamak 3, Zamak 5 | Painting, Chrome Plating, Nickel Plating, Phosphating |
| Nickel & Nickel-based Alloys |
Inconel 718, Monel 400 | Electropolishing, Passivation, PVD/CVD Coating |
High-quality metal CNC machining parts should match high-quality surface finishes to prevent oxidation and maintain both aesthetics and performance.
VMT's professional team possesses extensive experience in challenging surface finishes such as polishing, multi-color anodizing, painting, sandblasting, electroplating, and etching. We are always committed to innovating cutting-edge surface protection processes to ensure components meet stringent safety and quality industry standards. You will find the perfect metal surface treatment solution for your project at VMT. (VMT CNC machining factory also provides prototyping and production services for CNC machined metal parts).
If your project is seeking surface treatment solutions for metal parts or products, please contact VMT experts for a free consultation and quote.

Is there any metal oxidation in CMOS (complementary metal oxide semiconductor)?
No. The semiconductor silicon is oxidized rather than a metal. The core of CMOS technology lies in using the 'metal' (refers to electrical materials such as polycrystalline silicon and metal gates) and 'oxide' (refers to silicon dioxide insulating layer) for manufacturing transistor fabrication.
What happens when lead metal is oxidized?
When lead metal is oxidized, it forms grayish-white or white lead oxides (PbO, PbO₂, Pb₃O₄, etc.). These oxides weaken the metallic luster, but do not cause severe structural corrosion like iron.
What is a non metal oxide?
Non-metal oxides are compounds formed by the reaction of non-metals with oxygen (O₂).
How to remove oxides?
Oxides can be removed from metal surfaces using methods like abrasive cleaning, acid washing, or electrochemical treatments. Common techniques include using sandpaper, wire brushes, or chemical rust removers.
How to determine if a metal is oxidized?
You can determine if a metal is oxidized by inspecting its surface for discoloration, such as a reddish-brown color on iron or greenish patina on copper. Additionally, tarnishing or a dull finish may indicate oxidation.
How to remove oxides from metal surfaces?
To remove oxides, you can use abrasive pads, steel wool, or a chemical oxide remover. Vinegar, citric acid, or phosphoric acid are also effective for dissolving the oxide layer, especially for aluminum or copper.
What causes metal oxidation?
Metal oxidation occurs when a metal reacts with oxygen in the environment, forming a chemical compound, such as rust in iron or patina in copper. This is often accelerated by moisture, heat, or exposure to air.
Which metals are prone to oxidation?
Metals like iron, steel, copper, aluminum, and zinc are particularly prone to oxidation. Iron forms rust when exposed to moisture, while copper develops a green patina. However, stainless steel and gold are more resistant to oxidation.
