15 years one-stop China custom CNC machining parts factory
 4 |
Published by VMT at Dec 24 2025 | Reading Time:About 3 minutes
4 |
Published by VMT at Dec 24 2025 | Reading Time:About 3 minutes
When you work with silver—whether for casting, manufacturing, or CNC machining projects—you may worry about overheating it, damaging its structure, or increasing production costs. This uncertainty grows when you’re unsure about the exact melting temperature of silver. By understanding the silver melt point and the factors that influence it, you can control your process with confidence and avoid costly mistakes.
The melting point of silver is 961.8°C (1763.2°F or 1234.95 K or 1234.95 K), which is the temperature where solid silver becomes liquid. Silver alloys melt across a range defined by their solidus and liquidus temperatures, depending on composition. Knowing the exact silver melting temperature helps you control casting, welding, and manufacturing processes safely and efficiently.
Now that you understand the basic melting temperature of silver, it’s time to explore how different forms of silver behave under heat. By looking at pure silver, common silver alloys, and the factors that influence melting performance, you’ll gain the clarity you need to handle silver confidently in casting, machining, and manufacturing applications.
Tip: Before working with any silver material, check its grade or alloy type—each one melts differently and affects your heat settings.
The melting point of silver is 961.8°C (1763.2°F or 1234.95 K), which is the exact temperature where solid silver turns into liquid metal. This value applies to pure silver, while alloys melt across a range defined by their silver solidus temperature (start of melting) and silver liquidus temperature (fully liquid). Knowing the precise silver fusion point helps you manage heating, avoid overheating, and control processes such as casting, refining, and CNC machining part preparation.
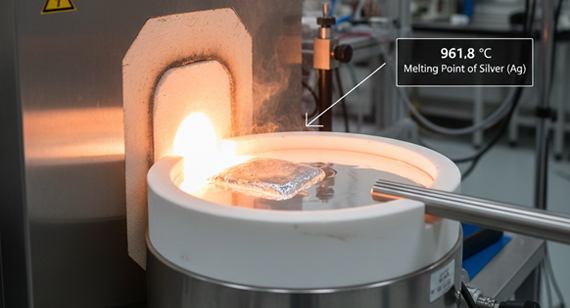
Silver melts at 961.8°C (1763.2°F or 1234.95 K) and boils at 2162°C (3924°F). The melting temperature of silver marks the point where solid silver turns to liquid, while the boiling point is when it becomes vapor. These thermal thresholds are crucial when you control heating during casting, refining, or preparing silver for manufacturing to avoid oxidation, overheating, or material loss.
Tip: Keep your working temperature slightly above the silver melt point—excessive heat wastes energy, increases oxidation risk, and can raise your overall project cost.
Understanding the melting point of silver helps you control temperature precisely during casting, welding, alloying, and heat-based manufacturing. When you know the silver fusion point and the melting point of silver alloys, you avoid overheating, reduce oxidation, and protect the metal’s mechanical properties. This knowledge also ensures consistent results in CNC machining part preparation, where heat exposure can affect dimensional stability and surface finish.
Different grades of silver melt at different temperatures because purity and alloying elements change how the metal behaves under heat. Pure silver has a fixed melting point, while alloys—including silver-copper, silver-zinc, silver-brass, and silver-gold—melt over a range defined by their solidus and liquidus temperatures. Understanding these values helps you choose the right heating settings for casting, refining, or manufacturing silver parts.
Melting Points of Silver Grades and Alloys
| Silver Type / Alloy | Composition | Melting Point / Range |
| Melting Points of Pure Silver (Ag) | 99.9%+ Ag | 961.8°C (1763.2°F or 1234.95 K) |
| Melting Points of Fine Silver | 99.9% Ag | 961.8°C (1763.2°F or 1234.95 K) |
| Melting Points of Sterling Silver | 92.5% Ag, 7.5% Cu | 893–965°C (1639–1769°F) |
| Melting Points of Coin Silver | ~90% Ag, 10% Cu | 875–970°C (1607–1778°F) |
| Melting Points of Silver-Based Alloys (General) | Ag with Cu, Zn, Ni, etc. | 700–1000°C (1292–1832°F) (varies by grade) |
| Melting Points of Silver-Copper Alloys | Ag-Cu in various ratios | 778–971°C (1432–1780°F) |
| Melting Points of Silver-Zinc Alloys | Ag-Zn | 650–900°C (1202–1652°F) |
| Melting Points of Silver-Brass Alloys | Ag with Cu + Zn | 760–950°C (1400–1742°F) |
| Melting Points of Silver-Gold Alloys | Ag-Au | 911–1060°C (1672–1940°F) depending on gold content |
Key Takeaways
Tip: When working with mixed silver alloys, heat too close to the liquidus temperature can cause uneven flow or grain growth—keep controlled, gradual heating to maintain part quality.
The silver melting process involves several controlled steps that help you heat the metal safely and efficiently. Each stage—preparation, heating, melting, pouring, cooling, and finishing—directly affects the final quality of the silver part. Understanding these steps allows you to avoid overheating, oxidation, and unnecessary material loss.
Preparation
You begin by cleaning the silver and removing contaminants such as oils, dirt, or oxidation. Proper preparation ensures even heating and prevents impurities from affecting the metal’s flow or final quality during casting or fabrication.
Heating
Heat is applied gradually using a furnace, torch, or induction heater. Increasing the temperature slowly reduces thermal shock and keeps the silver from oxidizing or forming unwanted surface defects. This is especially important when working with silver alloys that melt over a range.
Melting
Once the metal reaches its silver melt point (961.8°C / 1763.2°F or 1234.95 K for pure silver), it transitions into a liquid. For alloys, melting occurs between the solidus and liquidus temperatures, so the metal may start softening before fully liquefying. Proper temperature control ensures a clean, consistent melt.
Pouring
The molten silver is carefully poured into molds for casting. Smooth, controlled pouring prevents trapped air, incomplete fills, and surface defects. The mold temperature also matters—cool molds create rougher surfaces, while warm molds improve flow.
Cooling
After pouring, the silver cools and solidifies. Allowing it to cool naturally helps maintain its grain structure and reduces the chance of internal stress or cracking. Rapid cooling can warp the metal or make it brittle.
Finishing
Once the silver has cooled completely, you remove it from the mold and clean off oxidation, flux residues, or rough edges. Finishing steps may include grinding, polishing, or machining to achieve the final shape and surface quality.
The melting point of silver isn’t fixed—it changes depending on several intrinsic and extrinsic factors. Understanding these influences allows you to control the heating process accurately, avoid overheating or material defects, and ensure consistent results in casting, CNC machining parts, or alloy preparation.
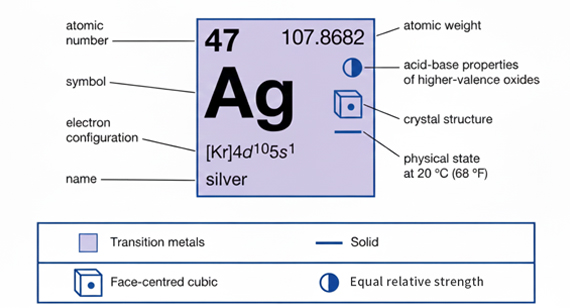
Atomic Structure of Silver
Silver’s melting behavior is influenced by its atomic structure. The arrangement of atoms in a crystal lattice determines how much energy is needed to break bonds and transition from solid to liquid. A stable lattice requires higher heat to melt.
Atomic Size
The size of silver atoms affects bond strength. Larger atomic spacing can slightly lower the melting temperature, while smaller atoms pack more tightly, requiring more heat to melt.
Purity of Silver
Purity is one of the most critical factors. Pure silver melts at 961.8°C (1763.2°F or 1234.95 K), but impurities like copper, nickel, or zinc lower or broaden the melting range. Impure silver may start melting at lower temperatures, which can affect flow and casting quality.
Alloying Elements
Adding elements like copper, zinc, or gold changes melting behavior. Some elements lower the melting point, while others may slightly raise it. This is why silver alloys have solidus and liquidus temperatures, creating a melting range rather than a single point.
Alloy Composition
Not just the presence, but the ratio of alloying elements matters. For example, silver-copper alloys melt differently depending on copper content. Precise composition control ensures predictable melting, better casting, and consistent CNC machining results.
Crystal Defects
Defects in the crystal lattice—like vacancies, dislocations, or inclusions—can reduce bond strength and slightly lower the melting temperature. High-quality silver with minimal defects melts more consistently.
Pressure
Applied pressure can influence melting behavior. Higher pressure generally raises the melting point, while reduced pressure lowers it. This is more relevant in industrial or vacuum processes than standard melting or casting.
Atmosphere
The surrounding environment affects melting. Oxidizing atmospheres can degrade silver or alloys during melting, while inert atmospheres help maintain purity. Proper flux use also protects against oxidation.
Particle Size (Applicable to Silver Powder)
Fine silver powders have higher surface area, which can lower the effective melting point compared to bulk silver. Smaller particles melt faster but are more prone to oxidation.
Understanding how silver’s melting point compares to other metals helps you choose appropriate materials for casting, CNC machining parts, or industrial processes. Metals like gold, copper, and aluminum melt at different temperatures, affecting heat control, alloying decisions, and workflow efficiency. The table below provides the melting points in Celsius, Fahrenheit, and Kelvin for easy reference.
Melting Points of Silver and Other Metals
| Metal | Melting Point (°C) | Melting Point (°F) | Melting Point (K) |
| Melting Points of Silver (Ag) | 961.8 | 1763.2 | 1235 |
| Melting Points of Gold (Au) | 1064 | 1947 | 1337 |
| Melting Points of Platinum (Pt) | 1768 | 3214 | 2041 |
| Melting Points of Brass | 900–940 | 1652–1724 | 1173–1213 |
| Melting Points of Copper (Cu) | 1085 | 1985 | 1358 |
| Melting Points of Bronze | 950–1050 | 1742–1922 | 1223–1323 |
| Melting Points of Palladium (Pd) | 1555 | 2831 | 1828 |
| Melting Points of Lead (Pb) | 327.5 | 621.5 | 600.6 |
| Melting Points of Steel | 1370–1510 | 2498–2750 | 1643–1783 |
| Melting Points of Stainless Steel | 1375–1530 | 2507–2786 | 1648–1803 |
| Melting Points of Iron (Fe) | 1538 | 2800 | 1811 |
| Melting Points of Aluminum (Al) | 660.3 | 1220.5 | 933.5 |
| Melting Points of Titanium (Ti) | 1668 | 3034 | 1941 |
| Melting Points of Tungsten (W) | 3422 | 6192 | 3695 |
| Melting Points of Tin (Sn) | 231.9 | 449.5 | 505 |
| Melting Points of Magnesium (Mg) | 650 | 1202 | 923 |
Key Takeaways
Tip: When combining silver with other metals, always consider their relative melting points to avoid partial melting or unwanted reactions during alloying.
Knowing the melting point of silver is essential for practical applications such as casting, welding, and annealing. Controlling temperature accurately ensures the metal flows correctly, maintains structural integrity, and avoids defects. Whether you’re creating jewelry, industrial components, or CNC machining parts, understanding silver’s thermal behavior is key to high-quality results.
In casting, silver is heated to just above its silver melt point (961.8°C / 1763.2°F or 1234.95 K) to ensure it flows smoothly into molds. For silver alloys, you must consider the solidus and liquidus temperatures, heating gradually to avoid uneven melting. Proper casting prevents porosity, surface defects, and dimensional inaccuracies.
Silver welding requires precise temperature control to avoid overheating the base metal. Using silver solder or alloy-specific welding rods, you heat just above the fusion point of the filler while protecting surrounding metal from oxidation. Accurate heat application ensures strong joints without compromising the surrounding material.
Annealing of Silver
Annealing involves heating silver to a temperature slightly below its melting point to relieve stress and improve ductility. This process softens the metal, making it easier to shape, form, or machine without cracking. For alloys, annealing temperatures must be adjusted based on composition to achieve uniform results.
The melting point of silver determines how you can use it across various applications, from jewelry to industrial components. Knowing its silver fusion point and the melting behavior of alloys allows you to choose appropriate processes for casting, forming, and machining. Accurate temperature control ensures high-quality results while minimizing waste and production costs.
Jewelry Making
In jewelry, silver is melted and cast into intricate designs. The precise silver melt point ensures smooth flow into molds, preventing air bubbles or defects. Alloys like sterling silver have slightly lower melting ranges, which jewelers must account for during casting and soldering.
Industry
Silver’s melting properties are leveraged in manufacturing components such as bearings, contacts, and high-precision CNC machining parts. Accurate control of temperature ensures that parts retain dimensional stability and surface quality during casting or alloying.
Silver is used in conductive applications such as switches, connectors, and printed circuit boards. Its melting behavior affects soldering and joining processes, where precise heat application is critical to avoid damaging sensitive components.
Education
In educational settings, silver’s melting point is used to demonstrate metal casting, alloy behavior, and thermal properties. Controlled experiments with pure silver or alloys teach students about metallurgy and CNC machining processes.
Other Applications
Silver’s melting characteristics are also applied in chemical instruments, photography, and coin production. Understanding the silver melting temperature helps maintain consistency and quality across these varied uses.
Controlling temperature is critical when melting silver or working with silver alloys. Accurate heat management ensures smooth flow, prevents oxidation, and maintains the mechanical properties of the metal. By using the right tools and techniques, you can achieve consistent results in casting, welding, annealing, and CNC machining parts.
Using a Digital Thermometer
A digital thermometer allows you to monitor silver’s temperature precisely, ensuring you reach the silver melt point without overheating. For alloys, you can track temperatures across the solidus and liquidus range, avoiding partial melting or excessive thermal stress.
Using Flux
Flux protects silver from oxidation and contamination during heating. It forms a barrier that prevents tarnishing and promotes uniform melting. Flux is especially useful when working with alloys, where uneven melting can cause defects.
Controlling Heat Application
Gradual and controlled heat application prevents thermal shock, overheating, or metal distortion. Whether using a torch, furnace, or induction heater, increase temperature slowly to maintain consistent melting, especially for silver alloys with wide melting ranges.
Understanding the melting point of silver and the factors that influence it is essential for anyone working with silver in casting, welding, annealing, or CNC machining parts. Pure silver melts at 961.8°C (1763.2°F), while alloys melt across a range depending on composition, purity, and environmental conditions. Accurate temperature control, proper preparation, and knowledge of alloy behavior ensure high-quality results, minimize defects, and reduce production costs. By mastering these principles, you can confidently handle silver in jewelry, industrial components, electronics, and educational applications, achieving consistent and reliable outcomes.
1. What is the melting point of silver solder?
Silver solder melts at a lower temperature than pure silver, typically 620–780°C (1148–1436°F) depending on the alloy composition. It is used for joining silver parts without melting the base metal.
2. What is the melting point of nickel silver?
Nickel silver, an alloy of copper, nickel, and zinc (with no actual silver), melts around 978–1050°C (1792–1922°F) depending on the specific alloy.
3. Which metal is the hardest to melt?
Metals like tungsten (3422°C / 6192°F) and rhenium (3186°C / 5767°F) are among the hardest to melt due to their extremely high melting points.
4. What metals cannot be melted? Which metals cannot be welded?
Most metals can technically be melted, but some (like tantalum carbide or certain refractory metals) require extreme conditions. Metals with extremely high melting points are difficult to weld without specialized equipment, such as tungsten or platinum-group metals.
5. Which melts first, silver or gold?
Silver melts at 961.8°C (1763.2°F or 1234.95 K), while gold melts at 1064°C (1947°F). Therefore, silver melts before gold.
6. How is silver melted for casting?
Silver is heated gradually to just above its melting point, often using a furnace, torch, or induction heater. Proper flux and temperature control ensure smooth flow into molds and prevent oxidation. For alloys, the solidus and liquidus temperatures must be considered.
7. Why does silver have a low melting point?
Silver has a relatively low melting point because of its atomic structure and metallic bonding. The energy required to break the bonds in its crystal lattice is lower than that of refractory metals like tungsten or platinum, making it easier to melt.
